1. LEAD

High-purity lead is less prone to corrosion and can better resist the effects of sulfation
High-purity lead is less prone to corrosion and can better resist the effects of sulfation:
Lead is the primary material used in car batteries, and it is essential to ensure that the lead used is of high purity. Pure lead is soft and malleable, which makes it easy to work with during the manufacturing process. Additionally, pure lead is highly resistant to corrosion, a critical feature in a car battery. In a car battery, the lead is used to form the plates, which are then coated with lead dioxide or sponge lead. The plates are submerged in an electrolyte solution of sulfuric acid and water, and they generate electricity through a chemical reaction. In addition, lead is an excellent conductor of electricity, making it ideal for use in batteries.
1). Effects of low quality lead:
However, not all lead is created equal, and the quality of the lead used in a car battery can significantly affect its performance. Low-quality lead can contain impurities such as antimony, arsenic, and copper, which can reduce the battery’s lifespan and increase the risk of failure. These impurities can cause a buildup of sulfates on the battery’s plates, reducing their capacity to generate electricity and leading to premature battery failure.
2) Benefits of good quality lead:
In contrast, high-quality lead is free of impurities and can significantly extend the life of a car battery. The purity of the lead used in a car battery is typically measured in terms of its chemical composition, with the highest purity being 99.99%. High-purity lead is less prone to corrosion and can better resist the effects of sulfation, making it more durable and long-lasting.
3). Sourcing of good quality lead:
The sourcing of the lead is also crucial in determining its quality. High-quality lead typically comes from reputable sources that use ethical and environmentally sustainable mining practices. This ensures that the lead is free of contaminants and meets strict quality standards.
To ensure the quality of lead used in their batteries, many manufacturers like Nyrstar, Teck, and gravita have implemented strict quality control measures. This includes using advanced testing methods to ensure that the lead is free of impurities and meets the required chemical composition standards. Additionally, manufacturers may also use additives and other treatments to enhance the performance and lifespan of their batteries.
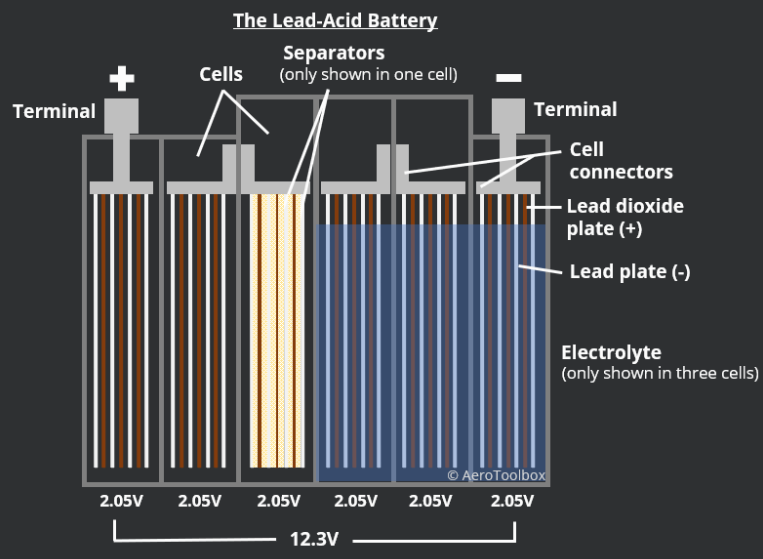
2. ELECTROLYTE
4 main factors for influcing the quality of Electrolyte: purity, concentration, temperature standards and additives.
4 main factors for influcing the quality of Electrolyte: purity, concentration, temperature standards and additives:
The electrolyte is a critical component of a car battery and plays a crucial role in its performance. It is the chemical solution that allows the battery to store and release electrical energy. In a lead-acid battery, the electrolyte is typically a solution of sulfuric acid and water. The quality of the electrolyte can have a significant impact on the battery’s lifespan and performance.
1). Purity:
One important consideration when choosing the electrolyte for a car battery is its purity. Impurities in the electrolyte can cause a variety of problems, including reduced battery capacity and increased corrosion. Manufacturers typically use high-purity sulfuric acid in the production of car batteries to ensure optimal performance and longevity.
2). Concentration:
Another critical factor to consider is the concentration of the electrolyte. In general, the higher the concentration of sulfuric acid in the electrolyte, the greater the battery’s capacity and performance. However, there is a tradeoff between concentration and battery lifespan. Concentrated electrolytes can be more corrosive and can accelerate the breakdown of the battery’s internal components over time.
3). Temperature Standards:
Manufacturers also need to consider the temperature at which the battery will be used when choosing the electrolyte. The electrolyte’s performance can be affected by temperature changes, and extreme temperatures can cause the electrolyte to freeze or boil. Manufacturers typically use additives in the electrolyte to improve its performance at both high and low temperatures.
4). Additives:
One common additive used in car battery electrolytes is antimony. Antimony helps to improve the battery’s performance at high temperatures by preventing the buildup of sulfate on the battery plates. However, there are concerns about the environmental and health impacts of antimony, and some battery manufacturers are exploring alternatives.
Other additives used in car battery electrolytes include calcium, which can improve battery capacity and lifespan, and magnesium, which can improve the battery’s ability to recharge. Manufacturers may also use organic additives, such as carboxylic acids, to improve the battery’s performance and reduce corrosion.
The electrolyte is a critical component of a car battery and can have a significant impact on its performance and lifespan. Manufacturers need to carefully consider the purity, concentration, and additives used in the electrolyte to ensure optimal performance in a variety of conditions. By selecting the right electrolyte and carefully managing its composition, manufacturers can create car batteries that are reliable, durable, and able to deliver consistent performance over time.

MAXTOP batteries are produced with high purity good quality lead and electrolyte with proper additives to ensure the longer-lasting power and higher durability
MAXTOP batteries are engineered to meet the highest industry standards, and our production process is closely monitored to ensure consistency in quality. The use of high-purity lead and electrolyte, coupled with proper additives, helps to guarantee that MAXTOP batteries deliver the reliable, long-lasting power required for today’s vehicles. With a focus on excellence and attention to detail, MAXTOP is a trusted name in the automotive battery industry.
In conclusion, a high-quality car battery relies on the use of high-quality raw materials. From the lead used in the grids and plates to the electrolyte and separators, every component plays a crucial role in the battery’s performance and lifespan. By choosing a reputable brand like MAXTOP that uses 4BS type plates and high-quality materials, you can ensure that your car battery will last longer and perform better, providing you with reliable and consistent power for years to come.
